Reforms do not always make things better
China has made great strides in implementing an agenda that began 41 years ago on reform and opening up. In particular, notable progress and improvements have been made in a variety of areas since China joined the World Trade Organization in 2001.
As the independent voice of European business in China since that same year, the European Chamber actively participates in the legislative process. The Chamber’s advocacy activities are widely recognised by the Chinese authorities and maintained through the efforts of its working groups and desks.
We launched the #becauseofus campaign to show our gratitude for the joint efforts made by all stakeholders: governments, think tanks, member companies and our working group and desk managers. The winning entry in this round, by Vöoker Müeller, Senior Business Manager, describes the positive impact of advocacy by the Chamber’s Healthcare Equipment Working Group.
#becauseofus
The ‘two-invoice’ how well-intended reform efforts may have unwanted side-effects that require the concerted efforts of all stakeholders, including those in the industry, to ward off calamities.
The distribution market for medical devices in China is in a precarious state. In late 2017, the number of trading companies for medium and high-risk medical devices in China reached 410,000;[1] this means on average 34 distributors for each public hospital. Many of the trading companies serve only a few hospitals, lacking qualified personnel as well as high-end means of transportation and storage. Because of hefty mark-ups in the supply chain, the prices of medical devices for hospitals in China are often much higher than in Europe. Despite strong efforts of the government to create a clean market, corruption is still widespread. To paraphrase an official: ‘It takes years and a huge amount of money to train a qualified medical doctor, and then we lose him, because we are not able to establish a clean, corruption-free environment.’
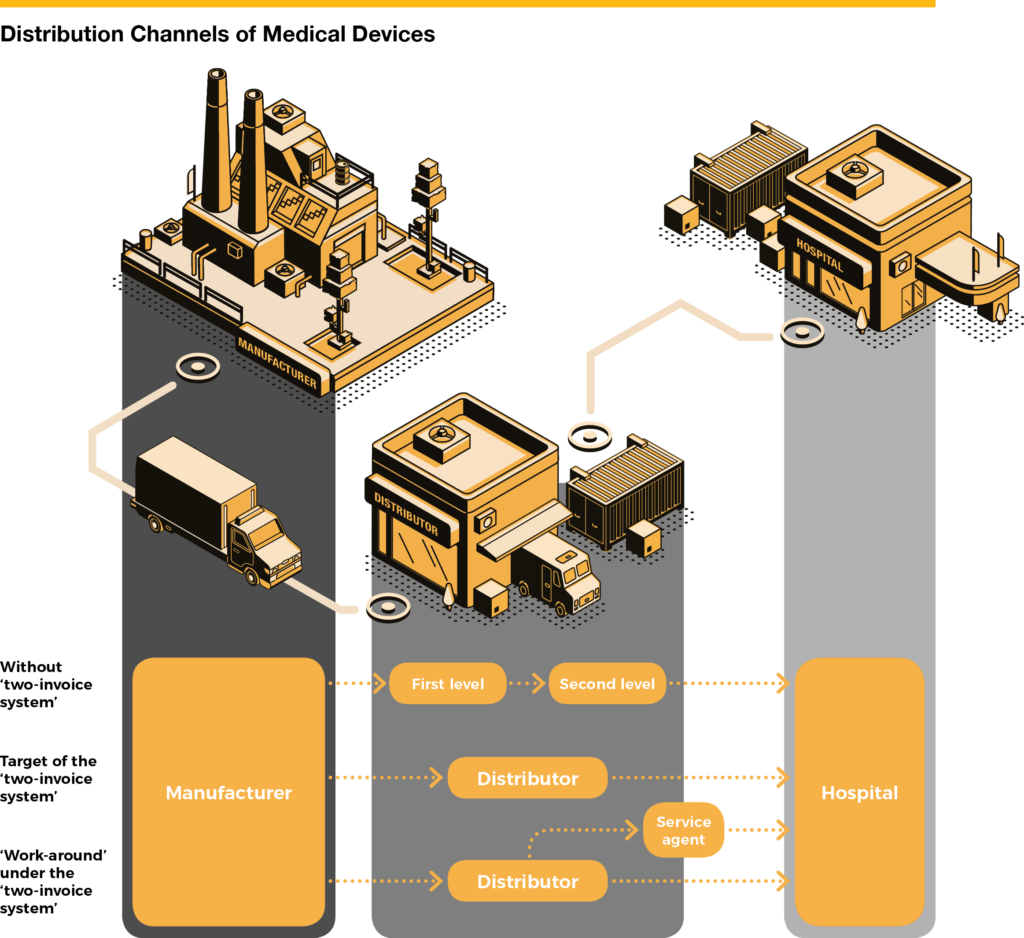
One of the government’s answers to this malaise is the ‘two-invoice system’, a unique regulation existing only in China. The name, the ‘two-invoice system’, is a direct translation of the Chinese ‘两票制’, a set of regulations that limits the number of distributors. The manufacturer (or in the case of imported products, the importer) sells their products to a distributor (first invoice), with the distributor then selling directly to the hospital (second invoice). According to the ‘two-invoice system’, secondary distributors or local dealers are not permitted. The first phase of the ‘two-invoice system’ saw it implemented for the distribution of pharmaceutical products, where it yielded some positive results. In the second phase, many provincial governments also wanted to apply the system to the medical device industry.
There is one big difference between pharmaceutical products and medical devices: medical device distributors are not just sales and logistics companies, but are also indispensable service providers for many types of complicated devices. Local distributors offer valuable services, like training for medical doctors, commissioning devices necessary for surgery, and the maintenance and repair of devices. In the case of implants, it is often the distributor who monitors whether the patient is doing well after being discharged from hospital. A distributor must undergo intense training by the manufacturer before it can qualify to deal with a device.
By definition, services need to be close to the customer. Several Chinese provinces are as large as major European countries, and in many cases a single distributor cannot provide high-quality service to all four corners of a province. In a few provinces where the government insisted on implementing the two-invoice system for medical devices, manufacturers came up with a ‘workaround’ of splitting delivery and service, employing one company complying with the two-invoice system for logistics and billing and another local company to provide the service. Obviously, this makes the whole supply system even more complex, and drives expenses up instead of limiting them.
The members of the European Chamber’s Healthcare Equipment Working Group analysed the difficulties of implementing the ‘two-invoice system’, submitted written recommendations to the authorities and held a series of meetings with provincial government agencies. Fortunately, most government officials proved very open to well-founded arguments and constructive recommendations, and did not extend the ‘two-invoice-system’ to medical devices.
Advocacy, if done well, may be a ‘win-win-win’ game, everyone wins; the government, the public healthcare insurance, the hospitals, the patients and finally the industry.
- 代理商解决医生和厂家都不愿意做的器械服务的问题,这些与我们毎个个体息息相关,所以希望都能改进。
- Distributors provide a necessary service that neither the doctors nor the manufacturers are able or willing to provide. This concerns the health of all of us; we hope to see even further improvement in the quality of service for medical devices.
- 医疗器械流通中两票制对于规范厂家与代理商的商业行为,简化及减少流通环节成本,净化商业环境,减轻患者与医疗机构的经济负担具有一定意义。但为了提高代理商的服务质量和专业化水平,加强售后服务,解决医院的实际需要,也有必要探讨对两票制的修正与改良。而欧盟商会在这其中做了大量卓有成效工作,这对于促进欧盟与中国贸易的良性发展及国家医改的意义是巨大且长远的。
- The ‘two-invoice system’ has positive effects on regulating the operation of medical device manufacturers and distributors. It can reduce costs in the distribution process and lower the economic burden of hospitals and patients. But in order to improve after-sales service quality, it’s important to re-evaluate the ‘two-invoice system’. The European Chamber contributed a lot towards solving this issue, this will have a long-term positive impact on Chinese trade and China’s healthcare reform.
[1] China Association of Medical Equipment, Development of the Medical Device Industry in China (in Chinese), People’s Medical Publishing House, Beijing, 2018, p. 16


Recent Comments